The National Ozone Unit Observed 2016 World Ozone Day with a series of activities geared toward the raising of awareness of the Montreal Protocol and the phase out of ozone depleting gases. Some of these included information sharing at the Grand Bazaar Mall (facilitated by Extra Foods Ltd.) and a seminar at Atlantic LNG.
RAC Technician Professionally Certified Registry
- Home
- Professional Certification for RAC Sector
- Updated Registry of Professionally Certified Refrigeration & Air-Conditioning Technicians in T&T
- RAC Technicians Corner
- Advisories
- General Documentation and Information for RAC Training Institutions
- Virtual Trainings and Webinars
- Rewards Programme for the Professional Certification Scheme for Refrigeration and Air Conditioning (RAC) Technicians
Tuesday, 20 September 2016
Friday, 16 September 2016
Ozone Maps (The Ozone Hole in 2016)
Ozone Hole September 2016
August 2016
July 2016
June 2016
The false-color view of the monthly-averaged total ozone over the Antarctic pole. The blue and purple colors are where there is the least ozone, and the yellows and reds are where there is more ozone.
The data is from the OMI instrument (KNMI / NASA) onboard the Aura satellite. They are the OMTO3d that have been processed in a manner similar to the TOMS data from earlier years.
Stratospheric “good” ozone
Ninety percent of the ozone in the atmosphere sits in the stratosphere, the layer of atmosphere between about 10 and 50 kilometers altitude. The natural level of ozone in the stratosphere is a result of a balance between sunlight that creates ozone and chemical reactions that destroy it. Ozone is created when the kind of oxygen we breathe—O2—is split apart by sunlight into single oxygen atoms. Single oxygen atoms can re-join to make O2, or they can join with O2 molecules to make ozone (O3). Ozone is destroyed when it reacts with molecules containing nitrogen, hydrogen, chlorine, or bromine. Some of the molecules that destroy ozone occur naturally, but people have created others.
The total mass of ozone in the atmosphere is about 3 billion metric tons. That may seem like a lot, but it is only 0.00006 percent of the atmosphere. The peak concentration of ozone occurs at an altitude of roughly 32 kilometers (20 miles) above the surface of the Earth. At that altitude, ozone concentration can be as high as 15 parts per million (0.0015 percent).
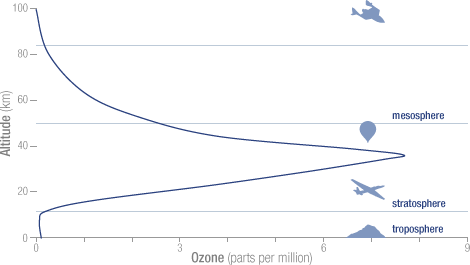 The concentration of ozone varies with altitude. Peak concentrations, an average of 8 molecules of ozone per million molecules in the atmosphere, occur between an altitude of 30 and 35 kilometers.
The concentration of ozone varies with altitude. Peak concentrations, an average of 8 molecules of ozone per million molecules in the atmosphere, occur between an altitude of 30 and 35 kilometers. Ozone in the stratosphere absorbs most of the ultraviolet radiation from the Sun. Without ozone, the Sun’s intense UV radiation would sterilize the Earth’s surface. Ozone screens all of the most energetic, UV-c, radiation, and most of the UV-b radiation. Ozone only screens about half of the UV-a radiation. Excessive UV-b and UV-a radiation can cause sunburn and can lead to skin cancer and eye damage.
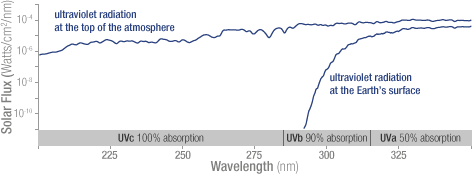 Solar ultraviolet radiation is largely absorbed by the ozone in the atmosphere—especially the harmful, high-energy UV-a and UV-b. The graph shows the flux (amount of energy flowing through an area) of solar ultraviolet radiation at the top of the atmosphere (top line) and at the Earth’s surface (lower line). The flux is shown on a logarithmic scale, so each tick mark on the y-axis indicates 10 times more energy.
Solar ultraviolet radiation is largely absorbed by the ozone in the atmosphere—especially the harmful, high-energy UV-a and UV-b. The graph shows the flux (amount of energy flowing through an area) of solar ultraviolet radiation at the top of the atmosphere (top line) and at the Earth’s surface (lower line). The flux is shown on a logarithmic scale, so each tick mark on the y-axis indicates 10 times more energy. Increased levels of human-produced gases such as CFCs (chlorofluorocarbons) have led to increased rates of ozone destruction, upsetting the natural balance of ozone and leading to reduced stratospheric ozone levels. These reduced ozone levels have increased the amount of harmful ultraviolet radiation reaching the Earth’s surface. When scientists talk about the ozone hole, they are talking about the destruction of stratospheric, “good,” ozone.
Tropospheric “bad” ozone
Although ozone high up in the stratosphere provides a shield to protect life on Earth, direct contact with ozone is harmful to both plants and animals (including humans). Ground-level, “bad,” ozone forms when nitrogen oxide gases from vehicle and industrial emissions react with volatile organic compounds (carbon-containing chemicals that evaporate easily into the air, such as paint thinners). In the troposphere near the Earth’s surface, the natural concentration of ozone is about 10 parts per billion (0.000001 percent). According to the Environmental Protection Agency, exposure to ozone levels of greater than 70 parts per billion for 8 hours or longer is unhealthy[1]. Such concentrations occur in or near cities during periods where the atmosphere is warm and stable. The harmful effects can include throat and lung irritation or aggravation of asthma or emphysema.The following link will take you to a non-NASA site.
[1] 2015 National Ambient Air Quality Standards (NAAQS) for Ozone.
Wednesday, 14 September 2016
Stakeholder Engagement Sessions for Enforcement of TTS 76 Part 20: Labelling of Containers of Refrigerants
The National Ozone Unit has been in collaboration with the Implementation Division, Trinidad and Tobago Bureau
of Standards (TTBS), with respect to stakeholder engagement sessions for the Enforcement of TTS 76 Part 20:
Labelling of Containers of Refrigerants. The purpose of these sessions is to continue discussion on the enforcement of TTS 76: Part 20: 2015.
Thus far sessions have been conducted on Wednesday 24th August, 2016 and Thursday 8th September, 2016.
The sessions were attended by member of the air conditioning and refrigeration importer community and there was consensus on the mechanisms for implementation of the Standard. Copies of the Standard can be obtained at the TTBS.
Tuesday, 13 September 2016
Summary of the HFC amendment proposals submitted by Canada, Mexico and the United States (North American proposal), India (Indian proposal), the European Union and its member States (European Union proposal) and some island States (Island States proposal)
There are a lot of current negotiations under the Montreal Protocol geared toward the inclusion of HFCs. The table below summarizes the various proposals.
http://ozone.unep.org/en/hfc-management-documents-2014-onwards
http://ozone.unep.org/en/hfc-management-documents-2014-onwards
Wednesday, 7 September 2016
NOU exhibits Hydrocarbon (HC) Refrigerator at the UTT Solar House
The Solar House at the University of Trinidad and Tobago (UTT) has a new edition. The National Ozone Unit has provided the UTT Point Lisas Campus Solar House with a Hydrocarbon Refrigerator for display and facilitation of further public education on this new technology. This and all other amenities in the House are powered by solar energy. UTT can be contacted for tours of the Solar House.
Tuesday, 6 September 2016
Thursday, 1 September 2016
Subscribe to:
Comments (Atom)


























